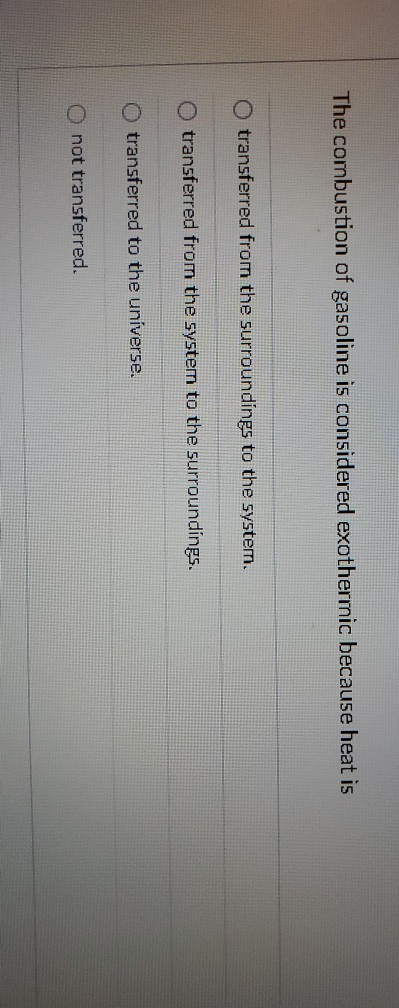
The Combustion of Gasoline Is Considered Exothermic Because Heat Is
When it is exposed to higher temperatures the dry ice changes directly from a solid to a gas. Combustion is an oxidation reaction that produces heat and it is therefore always exothermic.

Why Is Thermal Energy Released During A Combustion Reaction Quora
Natural gas is primarily methane CH 4.
. Take natural gas for example methane used to heat homes or used in stoves. Overall energy must be conserved so the excess energy of the products is usually released as heat. When coal is burned in a power plant __________ energy is converted into electricity a form of kinetic energy.
Are H 2 O g. In less common circumstances the reverse can happen. The main use of fuel combustion.
C6H12O6 6O2 6CO2 6H2O energy. Combustion reactions always involve molecular oxygen O2. During exothermic reactions like combustion bonds are broken which allows the energy trapped in the bonds to be released and do work.
Heat excites water molecules causing them to collide faster and change state from liquid to gas. The released energy is usually transferred as heat energy so one way to tell if a chemical reaction is. Combustion reaction produce products which have a lower energy state than the reactants which were present before the reaction.
On this basis it becomes apparent that combustions are exothermic because of the unusually small bond-dissociation energy of O2. Which has the lowest. Reacts oxygen with a fuel to produce carbon dioxide and water.
Exothermic The reacrion is exothermic or endothermic if the energy released in making the products is greater than the energy needed to break the bonds in the reactants. Dry ice the solid form of carbon dioxide has a lower temperature than ice. A common example is combustion of gasoline with oxygen to form water and carbon dioxide both of which have unusually strong bonds.
An exothermic process is one that gives off energyheat while an endothermic process absorbs heatenergySo a combustion reaction which is basically an explosion would definitely be exothermic. Both water and carbon dioxide are molecules which have less stored. Usually heat and light are released during a combustion reaction.
Usually the fuel that is combusted is a hydrocarbon that reacts with the oxygen in the air. F 2 g 2F g endothermic 13. Why is combustion considered to be an exothermic reaction.
Why is the reaction between methane and oxygen exothermic. It gives out a large amount of heat energy. The combustion of gasoline in a car engine exothermic.
When organic molecules combust the reaction products are carbon dioxide and water as well as heat. When the sugar burns by reacting with oxygen it produces mostly water and carbon dioxide. The heat content or the amount of energy produced when a fuel is burned is mainly determined by the carbon C and hydrogen H content of the fuel.
This is an exothermic reaction. Water condensing on a cold pipe exothermic g. Fuel combustion also known as burning fuel is the process by which a fuel is consumed in an exothermic chemical reaction that released a great deal of heat and light.
All chemical reactions first break bonds and then make new ones to form new materials. O transferred from the system to the surroundings. A combustion reaction is defined as the reaction in which hydrocarbons react with oxygen and results in the formation of carbon dioxide and water.
The combustion of gasoline is considered exothermic because heat is O transferred from the surroundings to the system. Which of the following has the highest enthalpy at a given temperature and pressure. The generated heat is used for purposes ranging from driving a motor cooking etc.
Combustion does not always result in fire because a flame is only visible when substances undergoing combustion vaporize but when it does a flame is a. Combustion reactions are almost always exothermic ie they give off heat. Anytime anything burns in the usual sense it is a combustion reaction.
The amount of CO 2 produced when a fuel is burned is a function of the carbon content of the fuel. The Burning of Hydrogen in Hydrogen-oxygen fuel cells is a type of exothermic reaction as it undergoes combustion and produces an electric charge. Write the balanced equation and draw Lewis structures of reactants and products to determine the number of CO bonds formed during the combustion of propane.
Also it is known that. Heat is produced when C and H combine with oxygen O during combustion. A significant amount of heat energy is required to trigger such a reaction.
All the combustion processes are exothermic because combustion increases the temperature where it occurs. H 2 O s. H 2 O l.
Burning release heat the molecular structure of methane is. Overall combustion is an exothermic reaction given off or exiting which means that energy is released. It means that enthalpy of reaction is negative.
Usually heat of combustion is considered to be a synonym of calorific value which can be defined as the total amount of energy liberated when a given mass of a substance undergoes complete combustion in the presence of an adequate quantity of oxygen under standard conditions for temperature and pressure. Transferred to the universe. Combustion reactions are always exothermic in nature.
The glucose on combustion with oxygen gives CO 2 gas H 2 O and evolves heat. You just studied 12 terms. Burning of Fuels whether it is a cars engine or a gas stove burning of fuels is also an exothermic reaction.
Lets think about what happens in the combustion of methane. TAP THE ARROWS BELOW TO ADVANCE. This reaction is called Respiration.
Respiration is another exothermic reaction and energy is released by this reaction. Combustion or burning is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant usually atmospheric oxygen that produces oxidized often gaseous products in a mixture termed as smoke. Is an exothermic reaction that releases energy in the forms of heat and light.
CLICK THE ARROWS BELOW TO ADVANCE. CO 2 s CO 2 g endothermic h. A fuel sugar for example has a great deal of chemical potential energy.
The combustion of gasoline in a car engine exothermic f.

Enthalpy Heat Combustion Experiment 4 Energy Level Heat Exothermic Reaction

Question Video Comparing The Heat Transferred During Combustion And Freezing Of Gasoline Nagwa

Solved The Combustion Of Gasoline Is Considered Exothermic Chegg Com
No comments for "The Combustion of Gasoline Is Considered Exothermic Because Heat Is"
Post a Comment